London, UK
Reuters
A cheap and widely used steroid called dexamethasone has become the first drug shown to be able to save the lives of COVID-19 patients in what scientists said is a “major breakthrough” in the coronavirus pandemic.
Trial results announced on Tuesday showed dexamethasone, which is used to reduce inflammation in other diseases such as arthritis, reduced death rates by around a third among the most severely ill COVID-19 patients admitted to hospital.
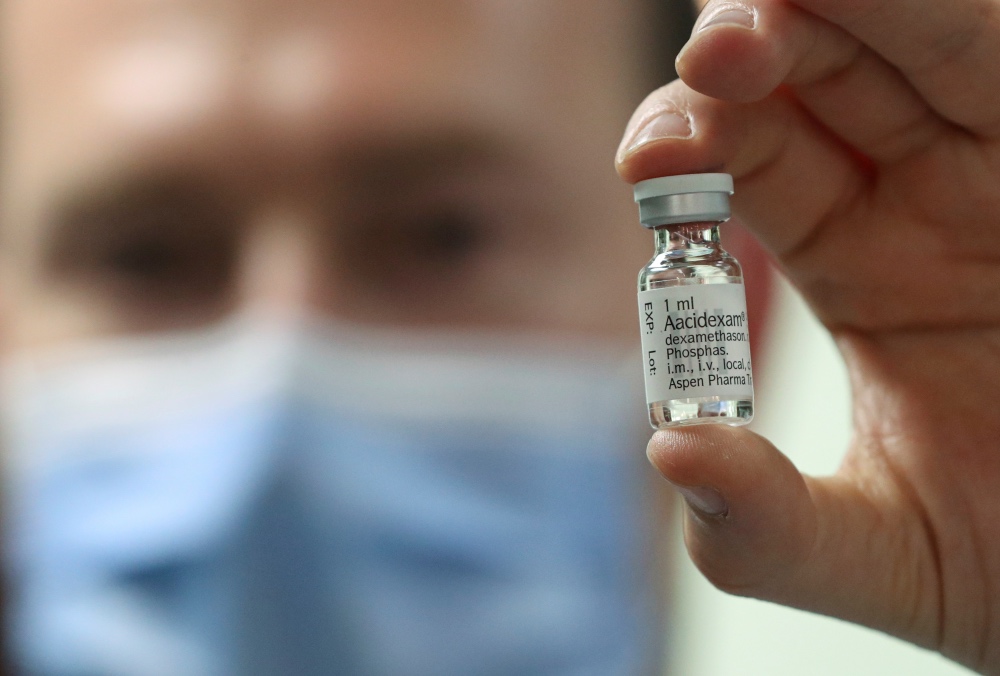
A pharmacist displays an ampoule of Dexamethasone at the Erasme Hospital amid the coronavirus disease (COVID-19) outbreak, in Brussels, Belgium, on 16th June. PICTURE: Reuters/Yves Herman
The preliminary results, which have not been peer-reviewed, suggest the drug should immediately become standard care in patients with severe cases of the pandemic disease, said the researchers who led the trials.
They said they would work to publish the full details of the trial as soon as possible, and many scientists said they hope to be able to review the evidence for themselves soon, especially given the recent retraction of an influential COVID-19 study.
UNDER 20S AROUND HALF AS SUSCEPTIBLE TO COVID-19, STUDY FINDS
People under 20 are around half as susceptible to COVID-19 as people aged 20 or above, according to research published on Tuesday, and clinical symptoms of the pandemic disease appear in only about a fifth of infections in children and teens.
The research, a modelling study using data from 32 locations China, Italy, Japan, Singapore, Canada and South Korea, found that by contrast, COVID-19 symptoms appear in 69 per cent of infections in people aged 70 or older.
The findings suggest that school closures – introduced in many countries as part of lockdowns aimed at controlling the coronavirus pandemic – are likely to have a limited impact on transmission of the disease, the researchers said.
Published in the journal Nature Medicine, the study compared the effect school closures on simulated outbreaks of flu – which is known to spread swiftly in children – and of COVID-19, the disease caused by the new coronavirus.
“For COVID-19, there was much less of an effect of school closures,” said Rosalind Eggo, an infectious disease modeller at The London School of Hygiene and Tropical Medicine who co-led the study.
She added, however, that the findings come from simulated outbreaks and need to be reinforced with real-world research.
Using demographic data from the six countries, as well as from six studies on estimated COVID-19 infection rates and symptom severity across different age groups, the model showed that people under 20 are about half as susceptible to COVID-19 as people over 20, and that among 10 to 19 year-olds, only 21 per cent of those infected had clinical symptoms.
The researchers also simulated COVID-19 epidemics in 146 capital cities around the world and found that the total expected number of clinical cases varied with median age.
“The age structure of a population can have a significant impact,” said Nicholas Davies, who co-led the work. “Countries with more young people may experience a lower burden of COVID-19.”
– KATE KELLAND, Reuters
Britain’s health ministry wasted no time, saying the drug had been approved for use in the state-run health service, export restrictions had been introduced and 200,000 courses of the treatment had been stockpiled.
“This is a [trial] result that shows that if patients who have COVID-19 and are on ventilators or are on oxygen are given dexamethasone, it will save lives, and it will do so at a remarkably low cost,” said Martin Landray, an Oxford University professor co-leading the trial, known as the RECOVERY trial.
“For less than £50, you can treat eight patients and save a life,” he said in an online briefing. One death would be prevented in every 25 COVID-19 patients on oxygen that received the drug, he calculated.
His co-lead investigator, Peter Horby, called dexamethasone “a major breakthrough.”
No treatment for COVID-19, the disease caused by the new coronavirus, which has killed more than 431,000 people globally, has been shown to reduce the mortality of the disease, although Gilead Sciences Inc’s remdesivir shortened the recovery time for hospital patients.
“This blows remdesivir out of the water in terms of the effect size and the kind of effect,” said Dr Mark Wurfel, professor of medicine at the University of Washington.
Wurfel cautioned that it is important that the data be released and reviewed, “but this magnitude of improvement in mortality for a critically ill population is about the largest effect size that we’ve ever seen,” he said.
Drug availability
The RECOVERY trial compared outcomes of around 2,100 patients who were randomly assigned to get the steroid, with those of around 4,300 patients who did not get it.
“We hope the data on which these results are based will be published as soon as possible so that doctors can confidently put the treatment into practice,” said Robin Ferner, honorary professor of clinical pharmacology at University of Birmingham.
Dr Thomas McGinn, deputy physician-in-chief at Northwell Health, New York’s largest healthcare system, told Reuters that physicians at Northwell hospitals have been using steroids on a case-by-case basis because they can suppress patients’ immune systems and possibly make them susceptible to other infections.
He said that if the data is peer-reviewed and legitimised, it could spread the use of steroids in the sickest COVID-19 patients.
“Across the country now intensivists have been using it based on their judgment calls. If this is legitimate, you may find…instead of say five out of 10 intensive-care COVID patients getting it, maybe everybody would get it,” McGinn said.
Dexamethasone is currently on the US Food and Drug Administration’s list of drugs in shortage. Still several suppliers including one of the largest – Germany’s Fresenius SE – say they have the drug on the hand.
“We will seek to increase supply volumes or even production to keep up sufficient deliveries,” a Fresenius spokesman told Reuters.
Among patients with COVID-19 who did not require respiratory support, there was no benefit from treatment with dexamethasone.
– Additional reporting by LUDWIG BERGER and MIKE ERMAN






