India on Friday became the fourth country to approve a coronavirus vaccine developed by Oxford University and AstraZeneca, authorising the vaccine’s rollout in coming weeks in the country with the second highest number of infections.
The following is what we know about the race to deliver vaccines to help end the coronavirus pandemic, which has killed more than 1.8 million people worldwide:
Who is furthest along?
US drugmaker Pfizer and German partner BioNTech have been the COVID-19 vaccine trailblazers.
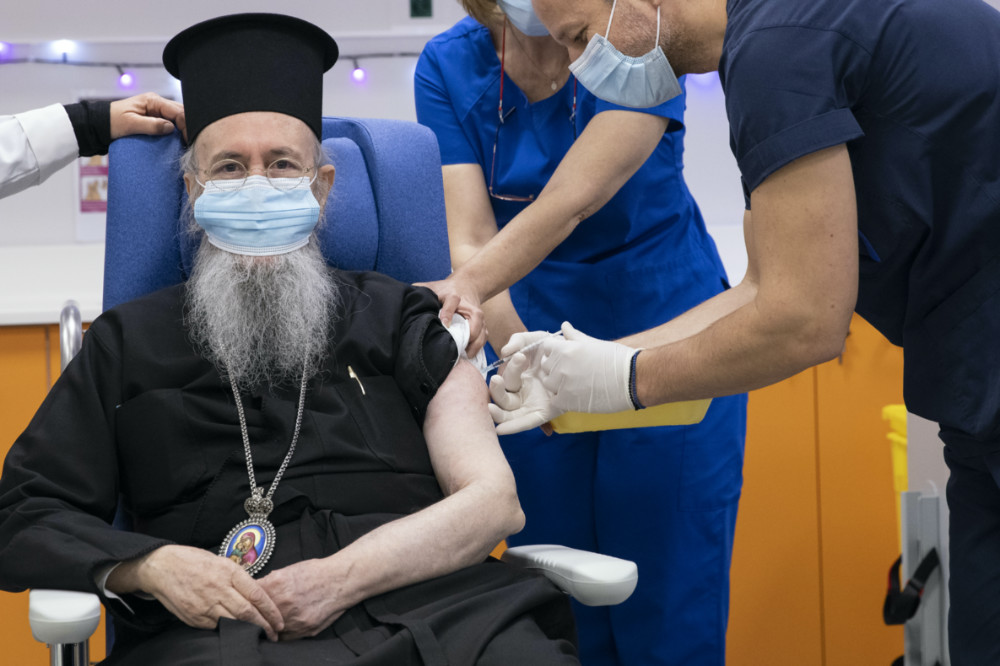
Metropolitan Hierotheos of Nafpaktos and Agios Vlasios receives an injection with a dose of COVID-19 vaccine, at Evangelismos hospital, in Athens, on Sunday, 27th December, 2020. Doctors, nurses and the elderly rolled up their sleeves across the European Union to receive the first doses of the coronavirus vaccine Sunday in a symbolic show of unity and moment of hope for a continent confronting its worst health care crisis in a century. PICTURE: Alkis Konstantinidis/Pool via AP.
On 18th November, they became the first in the world to release full late-stage trial data. Britain was the first to approve the shot for emergency use on 3rd December, followed by Canada on 9th December and the US Food and Drug Administration on 11th December. Several other countries, including Saudi Arabia and Mexico, have also approved it.
The European Medicines Agency approved the shot on 21st December and India is accelerating its review.
The World Health Organization on Thursday listed the vaccine for emergency use, in a move seeking to speed access to it in the developing world.
What about Moderna?
Moderna Inc was a close second to Pfizer in many countries after it released a full data analysis for a late-stage trial on 30th November showing a 94.1 per cent efficacy rate for its vaccine.
The United States authorised Moderna’s vaccine on 19th December, while Canada approved the shot on 23rd December and the European Medicines Agency will do so on 6th January.
AstraZeneca
India approval of a two-dose version of AstraZeneca’s vaccine, COVISHIELD, which has also been given the green light by Britain, Argentina and El Salvador, is a major win for a shot seen as crucial for mass immunisations.
Questions about the robustness of its trial data have complicated the approval process.
The British company announced interim late-stage trial data in November showing two full doses were 62 per cent effective while a half-dose followed by a full dose had a 90 per cent success rate – but UK regulators the more successful outcome had not stood up to analysis.
AstraZeneca is also in discussions with the European Union’s European Medicines Agency, which is conducting a rolling review of the vaccine.
Who else is in the running?
US drugmaker Johnson & Johnson plans to deliver trial data in January, teeing it up for US authorisation in February if its shot is effective. It reduced the enrolment target for its clinical trial to 40,000 volunteers from 60,000 on 9th December, potentially speeding results that are tied to how quickly participants become infected.
US firm Novavax is running a late-stage trial in Britain with data due in the first quarter of 2021. It expects to start a large-scale trial in the United States this month.
France’s Sanofi and Britain’s GlaxoSmithKline, however, announced a setback on 11th December in their attempts to develop a vaccine. The drugmakers said that it showed an insufficient immune response in older people in mid-stage trials and that they would start a new study in February.
What happens in the trials?
The companies usually test their vaccines against a placebo – typically saline solution – in healthy volunteers to see whether the rate of COVID-19 infection among those who got the vaccine is significantly lower than in those who received the dummy shot.
How are volunteers infected?
The trials rely on subjects becoming naturally infected with COVID-19, so how long it takes to generate results largely depends on how pervasive the virus is where trials are being conducted. Each drugmaker has targeted a specific number of infections to trigger a first analysis of their data.
How well are the vaccines supposed to work?
The World Health Organization ideally wants to see at least 70 per cent efficacy. The FDA wants at least 50 per cent – which means there must be at least twice as many infections among volunteers who received a placebo as among those in the vaccine group. The EMA has said it may accept a lower efficacy level.
What about Russia and China?
Although Pfizer’s shot was the first to be rolled out following the publication of full Phase III trial data, Russia and China have been inoculating their citizens for months with several different vaccines still undergoing late-stage trials.
China on 31st December approved its first COVID-19 vaccine for general public use, a shot developed by an affiliate of state-backed pharmaceutical giant Sinopharm. The company said it is 79 per cent effective against the virus.
Russia said on 24th November its Sputnik V vaccine, developed by the Gamaleya Institute, was 91.4 per cent effective based on interim late-stage trial results. It started vaccinations in August and has inoculated more than 100,000 people so far.
India plans to make 300 million doses of Sputnik V next year and Argentina has given the green light for emergency use of the shot, with some 300,000 doses arriving in the country on 24th December.
China launched an emergency use program in July aimed at essential workers and others at high risk of infection. It has vaccinated about one million people as of mid-November using at least three shots – one developed by the state-backed China National Biotec Group and one by Sinovac Biotech.
Trial data on a COVID-19 vaccine developed by China’s Sinovac Biotech has varied: interim data from a late-stage trial in Turkey showed its CoronaVac shot is 91.25 per cent effective, while researchers in Brazil say the shot was more than 50 per cent effective.
The United Arab Emirates, meanwhile, said on 9th December that one of the CNBG vaccines was 86 per cent effective based on interim results from a late-stage trial in the Gulf Arab state.





